Nuclides: Isotope, Isotones, Isobars and Isoelectronics
Learning goals:
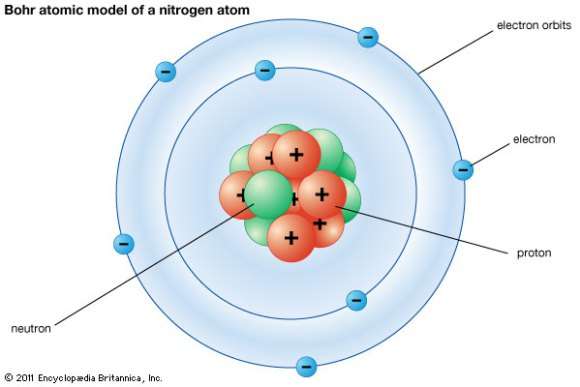
Students should be able to explain the concept of nuclides and differentiate between isotopes, isobars, and isotones.
Students should be able to interpret and use nuclear notation to represent nuclides, including the notation for atomic number, mass number, and specific isotopes.
Develop an understanding of the distinction between stable nuclides and radioactive nuclides, including knowledge of decay modes such as alpha decay, beta decay, and gamma decay.
Resources:
https://classroom.google.com/c/NjA4NDUxOTg5ODk4/a/NTU0NTM2Nzk4MzUy/details
https://classroom.google.com/c/NjA4NDUxOTg5ODk4/m/NjE1MjkzNTA5NzA1/details
https://classroom.google.com/c/NjA4NDUxOTg5ODk4/m/NTUzOTQyMzMzODQ2/details
Learning activities:
Learning by doing: Ss will make models of different isotopes using clay and recognize their differences. They will watch some videos related to radioactivity and nuclear fission.
Problem solved learning: Ss will resolve exercises about the determination of subatomic particles in isotopes, isobars, isotones and isoelectronics, they will also identify them.
